Packing Types
1. Side by side packing Expanding of side by side packing:
Expanding of side by side packing:
a. Simple (Primitive) Cubic
Again not close packed - primitive or simple cubic cell with atoms only at the corners. # atoms/unit cell = 1. A very rare packing arrangement for metals, one example is a form of Polonium (Po).
b. Body centered cubic
Not close packed - atoms at corners and body center of cube. # atoms/unit cell = 2.
2. Hexagonal Packing/Close packing
Hexagonal Close Packing. (HCP)
To build our 3-dimensional metal structures we now need to stack the close packed layers on top of each other. There are several ways of doing this. The most efficient space saving way is to have the spheres in one layer fit into the "holes" of the layer below.If we call the first layer "A", then the second layer ("B") is positioned as shown on the left of the diagram below. The third layer can then be added in two ways. In the first way the third layer fits into the holes of the B layer such that the atoms lie above those in layer A. By repeating this arrangement one obtains ABABAB... stacking or hexagonal close packing.

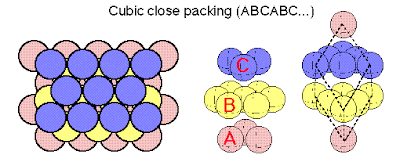
Cubic Close Packing (CCP)
While for the HCP structure the third close packed layer was positioned above the first , an alternate method of stacking is to place the third layer such that it lies in an unique position, in this way an "ABCABC..." close packed layer sequence can be created, see below. This method of stacking is call Cubic Close Packing (ccp)






0 comments:
Post a Comment
Untuk pembaca yang menginginkan pembahasan atau kunci jawaban dari post soal silahkan wa 08562908044 (fast respond) | monggo tinggalkan kritik, saran, komentar atau apapun ^_^